valence electron of cr|chromium unpaired electrons : Cebu The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements . Tingnan ang higit pa Share; Tweet; Reddit; Save; Post; As one of the more unique entries in the series, Mega Man 8 can be a tricky game so here are the best strategies for facing its bosses. │ Just like with PlayStation 5, Video Chums utilizes an SSD on our server to deliver lightning-fast page load times. ⚡ Mega Man 8 Boss Order / Weaknesses
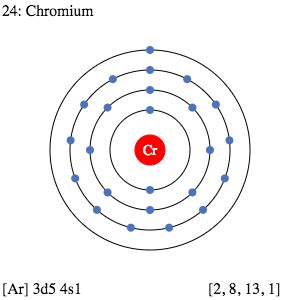
valence electron of cr,The 1st element in group-6 is chromium. The elements in groups 3-12 are called transition elements. The valence electrons are the total number of electrons in the last orbit. But in the case of transition elements, the valence electrons remain in the inner shell(orbit). Because the electron configuration . Tingnan ang higit paThe valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron . Tingnan ang higit paThe elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements . Tingnan ang higit pavalence electron of crThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some rules for diagnosing valency. The number of electrons in an unpaired state in the last orbital after the electron configuration . Tingnan ang higit pa Mar 23, 2023 To find the number of valence electrons for Chromium (Cr) we need to look at its electron configuration. This is necessary because Cr is a transition metal (d block element) and . You may assume the valences of the chemical elements—the number of .How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for . Explanation: Chromium lies in Group 6 of the Periodic Table; i.e. there are 6 valence electrons ... Given Cr, Z = 24, we could write the d-electron configuration as . The valence electrons of chromium include its 4s and 3d electrons, because they are close enough in energy that more than one electron can be used to bond. Its electron configuration as an atom is .

Chromium is a chemical element of the periodic table with chemical symbol Cr and atomic number 24 with an atomic weight of 51.9962 u and is classed as transition metal and is .Chromium is a chemical element of the periodic table with chemical symbol Cr and atomic number 24 with an atomic weight of 51.9962 u and is classed as transition metal and is .
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its .valence electron of cr chromium unpaired electrons Cr and Cu are the two exceptions of electron configuration of atoms up to Kr. this is because a 1/2 or completely full D block has extra stability, therefore in the case of . Valency of Chromium. Well, the valency of Chromium may be either of 2 or 3 as per the prevailing state. The valency of Chromium varies because it’s a transition state. Being the transition element, it is . Systematic differences in Cr-L 2,3 fine structure are apparent in the spectra of the oxidation state standards. For example, the Cr-L 2,3 edges for the standards show a systematic shift in peak position to higher energy with increasing valence from Cr(I) to Cr(VI). The peak height of the L 3 edge relative to the L 2 edge generally decreased with . Surely there are 6...? Chromium lies in Group 6 of the Periodic Table; i.e. there are 6 valence electrons... Given Cr, Z=24, we could write the "d-electron configuration" as follows... 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(4) At undergraduate level you learn that the electron configuration is actually...
You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.Inner electrons are determined via atomic number of the given atom along with the valence electron of that atom. The formula which is required for the evaluation of inner electron is given below. I n n e r e l e c t r o n s = A t o m i c n u m b e r .
For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. This is important as it is the Valence electrons 3d5 4s1, electrons in the outermost shell that determine the chemical properties of the element. Unabbreviated electronic configuration of neutral Chromium
To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number.valence electron, any of the fundamental negatively charged particles in the outermost region of atoms that enters into the formation of chemical bonds.Whatever the type of chemical bond (ionic, covalent, metallic) between atoms, changes in the atomic structure are restricted to the outermost, or valence, electrons.They are more weakly attracted to . To write the orbital diagram of chromium, you have to write the orbital notation of chromium. Which has been discussed in detail above. Orbital Diagram for Chromium. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electrons will first enter the 1s orbital. Valence electrons. Electron configurations of ions. Electron configurations of the 3d transition metals. Atomic structure and electron configuration. . Cr and Cu are the two . We know that Chromium has an atomic number of 24 so the first two electrons of Chromium will shift to the 1s orbital. The 1s orbital can hold only 1 electron therefore the other 2 electrons will move on to the 2s orbital. The next six electrons will then move to the 2p orbital. In a similar manner, the 3s orbital will have 2 electrons and .

Periodic Trends in the Oxidation States of Elements 1. Variation Of Oxidation State Along a Period. While moving left to right across a period, the number of valence electrons of elements increases and varies .The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the periodic table consists of atoms, which are composed of protons .
The valence electron configurations of the first-row transition metals are given in Table \(\PageIndex{1}\). As we go across the row from left to right, electrons are added to the 3d subshell to neutralize the increase in the positive charge of the nucleus as the atomic number increases. . Ti 4 +, Cr 3 +, and Ni 2 + in order of increasing .chromium unpaired electrons the valence electrons in Cr(CO)6. We denote the calculations as "valence only" if. only the valence electrons were correlated, or "3s3p-4-valence" if both the 3s3p and. valence electrons are .
Problems. State the oxidation state of the metal and the total valence electron count of the following species. 1. V(C 2 O 4) 3 3−. Ans: +3 and 14 2. Mn(acac) 3 Ans: +3 and 16 3. W(CN) 8 3− Ans: +5 and 17 4. CpMn(CO) 3 Ans: 0 and 18 5. Fe 2 (CO) 9 Ans: 0 and 18 Self Assessment test. State the oxidation state of the metal and the total valence electron . Recently, Nygard et al. [21] suggests that hydrogen sorption properties in BCC multicomponent alloys might be related to the valence electron concentration (VEC), defined by Eq. (1). (1) VEC = ∑ i = 1 N c i VEC i where c i is the atomic fraction of each element i with valence-electron concentration VEC i in a multicomponent alloy with N .
valence electron of cr|chromium unpaired electrons
PH0 · valence electrons of chromium
PH1 · electron dot diagram for chromium
PH2 · cr valence electron configuration
PH3 · cr unpaired electrons
PH4 · cr element valency
PH5 · chromium unpaired electrons
PH6 · chromium protons neutrons electrons
PH7 · chromium electrons
PH8 · Iba pa